Determine the Value and Units of the Rate Constant
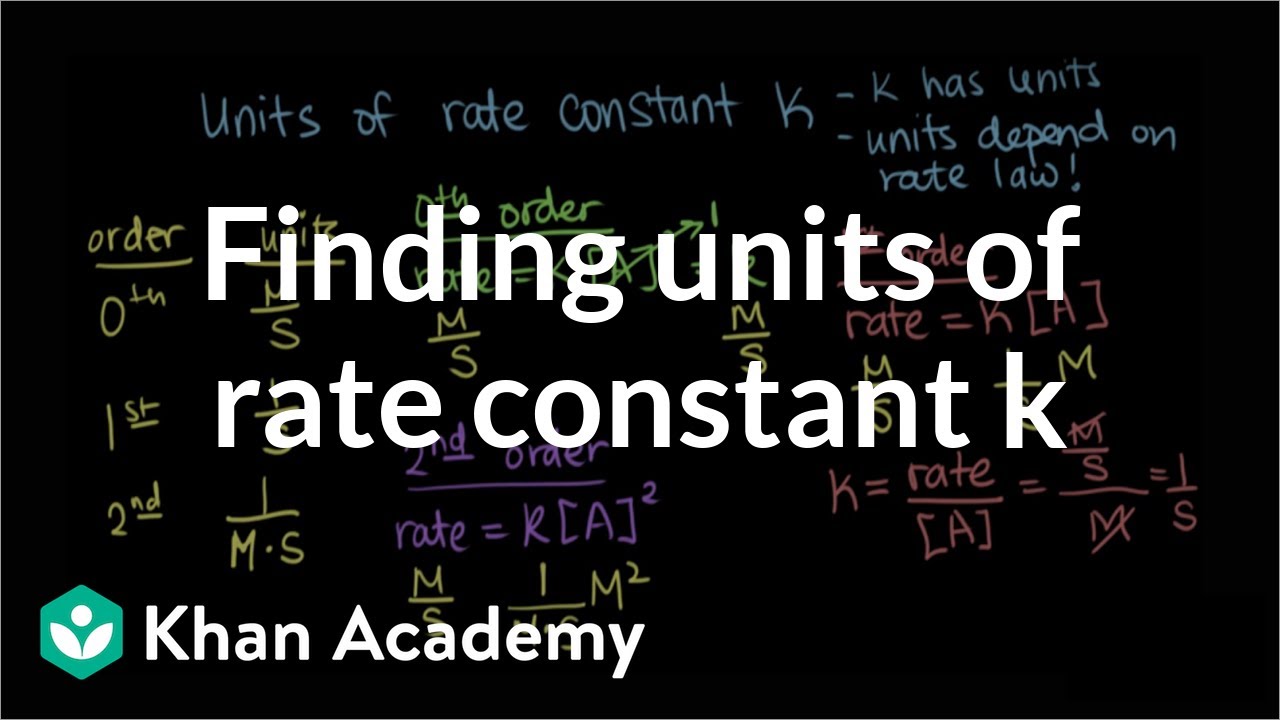
Rate k A 2 the units of rate are molL -1s -1 and the units of A concentration are always molL -1 units have to be the same on each side. First Order Reactions rate kA Mt k M k units.

Calculating The Rate Constant Using Initial Rates Data Example Pt 2of 3 Youtube
That causes the rate of reaction to almost double.

. 2 A 2 B 2 2 C b. Under the conditions specified in part e what would be the. Mol 1 m n L m n 1 s 1.
Determine the numerical value of the rate constant k with appropriate units. A is the frequency factor which has the same units as the rate constant. Rate constant from the Arrhenius equation.
Determine the value and units of the rate constant. 𝑘 667 10 30 units. Hence it is expressed in many units.
Third Order Reactions rate kA 3 rate kA 2 B rate kABC. S-1 min-1 hr-1 etc. For a zero order reaction the rate constant has units molar per second Ms or mole per liter per second molL 1 s 1 For a first order reaction the rate constant has units of per second of s -1.
For the given rate law determine the units of the rate constant for rate k A 2 B. R a t e A a B b. The units for the rate of a reaction are molLs.
A 2 B 2 R C slow A 2 R C fast a. This chemistry video tutorial explains how to determine the units of the rate constant K for a first order reaction second order reaction and a zero order. You can see that the fraction of the molecules able to react has almost doubled by increasing the temperature by 10C.
Include the correct units. Ms Mmin Mhr etc. Write the overall balanced chemical equation.
To use this online calculator for Rate constant of first order reaction enter Initial concentration C0 Amount reacted in time t x Reaction Time t and hit the calculate button. X 2 x 2. Following are the ways to express rate constant.
Where k is the rate constant. Gas Constant In Different Units. The k k s cancel out.
Identify any intermediates within the mechanism. For the following reaction aA bB products the generalised rate equation is. The value of the gas constant R is 831 J K-1 mol-1.
M-1 s-1 M-1 min-1 M-1 hr-1 etc. MolL -1s -1 k molL -1 2 therefore k units are Lmol -1s -1 Top. The gas constant is inversely used in diverse disciplines.
Here is how the Rate constant of first order reaction calculation can be explained with given input values - 0040547 ln 03 03-0110. R kAxBy r k A x B y. The rate is second order in respect to NOBr N O B r and the rate law is written rate kNOBr2 rate k N O B r 2.
A and b are the order of the reaction. In this equation A and B express the concentrations of A and B respectively in units of moles per liter. By raising the temperature just a little bit to 303 K this increases.
The unit for the rate constant is calculated from the rate law. Rate Constants for Common Reaction Orders. Write the complete rate law expression for the formation of I3-.
If you double the concentration the rate will quadruple. What would be the initial rate of formation of I3- the units of Ms when the following initial concentrations were used. Rate Constant k has UNITS.
In general for a reaction with order a b the units of the rate constant are mol 1 mn L mn1 s 1. Plug and chug using the rate law data from expt 1 and solving for k we get k 236 molL-1. K A e E a R T.
Determine the value of the rate constant including units for the formation of I3-. More generally speaking the units for the rate constant for a reaction of order m n m n are mol1mn Lmn1 s1. E_a is the activation energy usually in kJmol.
Solution for b Calculate the value of the rate constant k for the reaction. This is the value in the rule-of-thumb often used. The table below summarizes the rate constant units for common reaction orders.
Shields demonstrates how to calculate the value for the rate constant with units after determining the orders of each reactant using initial rates expe. R kAmBn r is used as symbol for rate The unit of r is usually mol dm-3 s-1 The square brackets A means the concentration of A unit mol dm-3 k is called the rate constant. Now solve for the units of k.
K Ae-E_aRT what is the equation called k is the rate constant. A and B are the molar concentration of reactants A and B. Thus gas constant R value can be given as Gas constant R 8314459848 Jmol 1 K 1.
K 948 M-1s-1 The relation between the rate constant and temperature is something you should be getting to know really well at this point. At 20C 293 K the value of the fraction is. For the general reaction aAbB C aA bB C with no intermediate steps in its reaction mechanism meaning that it is an elementary reaction the rate law is given by.
225 15x 225 15 x. 243 102 108 102 0900x 0600x 243 10 2 108 10 2 0900 x 0600 x. Consider the following mechanism.
Determine the value and units of the rate constant k. The units for k are whatever is needed so that substituting into the rate law expression affords the appropriate units for the rate. The digits inside the parentheses are the uncertainty in the measurement of gas constant value.
Zero Order Reactions rate kA 0 Mt k k units. The rate equation relates mathematically the rate of reaction to the concentration of the reactants. Second Order Reactions rate kA 2 rate kAB Mt k M 2 k units.
Units Of The Rate Constant Video Khan Academy

How To Determine The Units Of The Rate Constant K Chemical Kinetics Youtube
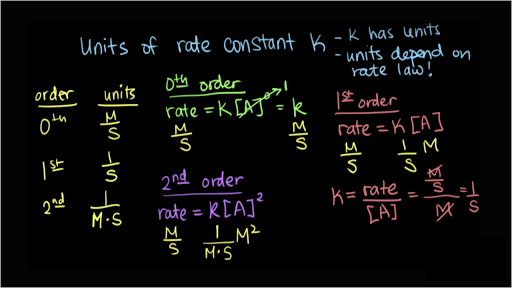
Comments
Post a Comment